For Providers
One clinically validated¹ mail-in semen analysis with a world of benefits
Fellow's mail-in semen analysis tests optimize workflows for your clinic and staff, improve the patient experience, and yield valuable insights into your patient's health and fertility.
Working With Us
Clinics that work with Fellow report:
How It Works
Offer patients a more comfortable testing experience
Trusted by over 3,000 urologists and reproductive specialists
Our Clinic Portal
A comprehensive view of your patient's testing journey
Our clinic portal saves you time and hassle by giving you a way to view your patients' testing progress and results.
Our Support Team
Dedicated support at every step
Our sales and support teams work closely with you to be sure your clinic has everything it needs to successfully offer patients a smooth, positive experience—and to answer questions if you need help.
Clinically validated¹ analysis powered by Fellow's innovative mail-in kit.
Our process is peer-reviewed to provide clinically valid results for samples produced up to 52 hours before they reach our lab.¹ Our proprietary algorithm accounts for sperm's natural rate of degradation over time, allowing us to calculate a sample's metrics just as accurately as if it had been produced and analyzed within 1 hour of ejaculation.
Clinically validated accuracy
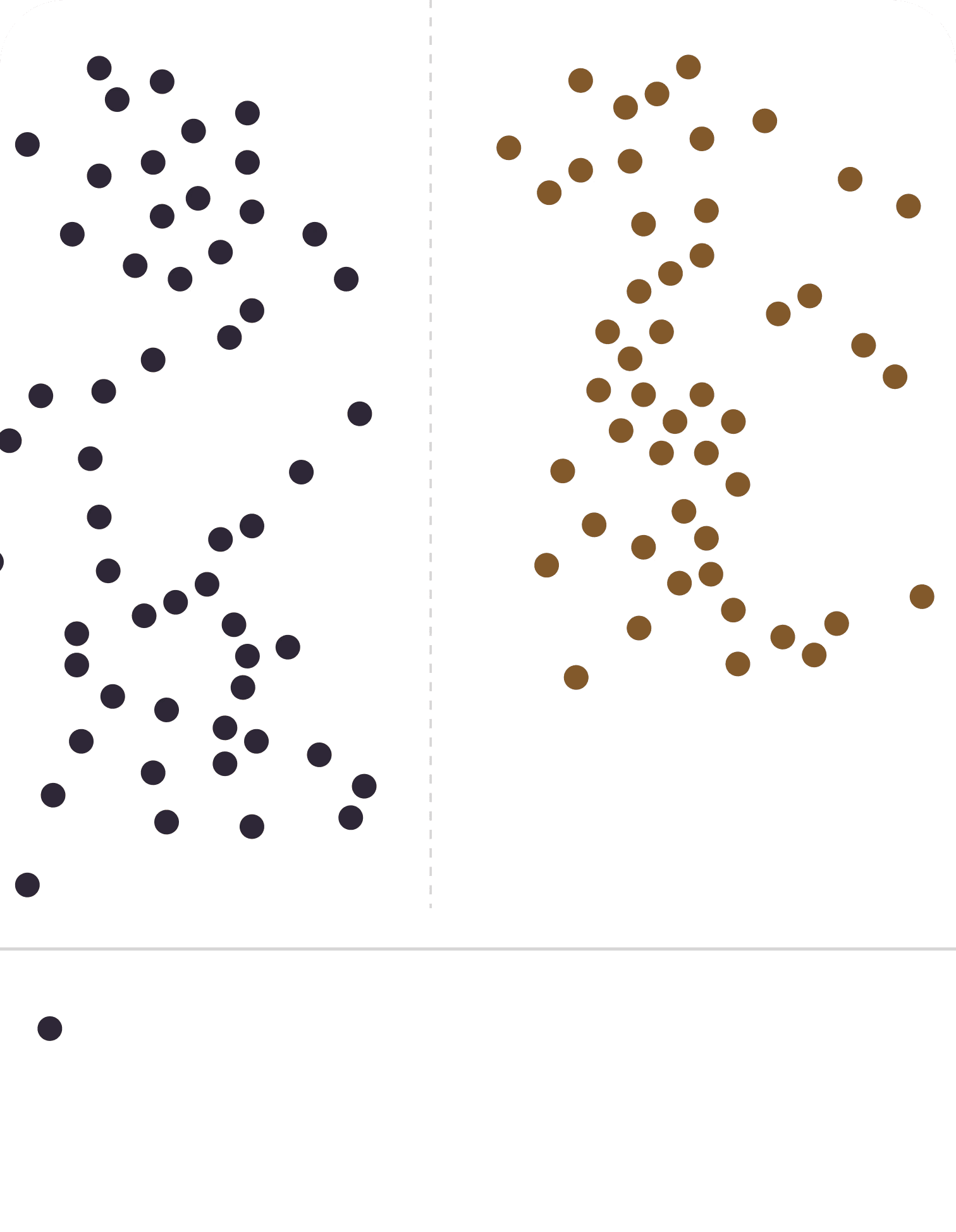
Fellow's validation study, published in the peer-reviewed journal Fertility and Sterility, demonstrated the accuracy of the Fellow test. Our mail-in semen analysis testing system has a strong degree of correlation between 1-hour and delayed SA testing. This test may be used in clinical practice to evaluate semen quality for fertility evaluations.
Development and validation of a novel mail-in semen analysis system and the correlation between one hour and delayed semen analysis testing

Correlation of fellow and gold standard
Independently reviewed
An independent review of our validation study noted the benefits of Fellow 's accurate mail-in test:
"The ability to perform at-home semen analyses testing has numerous clinical and patient implications, including reducing patient anxiety, improving convenience, and potentially the ability to see higher levels of patient compliance."
—Thomas A. Masterson, M.D. & Premal Patel, M.D.
Clinical implications of home-based sperm testing
The ability to perform at-home semen analysis testing has numerous clinical and patient implications, including reducing patient anxiety, improving convenience, and potentially the ability to see higher levels of patient compliance (3).
Further, another clinically important implication of at-home testing is the potential for better standardization of results. Routine semen analysis is fraught with interobserver variability. As such, a centralized location for evaluation can minimize interobserver variability and, thus, improve the diagnostic utility of the test results
Our Vision
We see a routine test as a potential
canary in a coal mine
With patient consent, each test contributes to a biobank of semen samples that can be used to yield breakthroughs across medical disciplines. In partnership with leading experts and universities, we pioneer research in male reproductive sciences. Read our abstracts and articles here.
Work With Us
Working with Fellow is simple
and convenient
We offer urologists, fertility specialists, and OBGYNs around the country a variety of options to make our tests accessible to patients. Request more information to hear from one of our local representatives, or write us directly at sales@meetfellow.com.
Samplaski et al. Development and validation of a novel mail-in semen analysis system and the correlation between one hour and delayed semen analysis testing; Fertil Steril. 2021;115(4):922-929
Tolani et al. Mail-in semen analysis breaks down racial, educational, and income barriers to semen analysis completion. Proceedings from the Pacific Coast Reproductive Society (PCRS) Annual Meeting 2024. Indian Wells, CA.
Gu et al. High compliance with Fellow semen analysis testing across the United States irrespective of clinical practice type. Proceedings from the American Urological Association Annual Meeting 2024. San Antonio, TX.
Multiple publications report only 1 in 2 patients complete post-vasectomy semen analysis, including Bradshaw et al., Urology, 2019, Duplisea and Whelan, Journal of Urology, 2013 and Johnson (Siegert) et al., Andrology & Gynecology: Current Research, 2019.